Driving Drug Commercialization with Oracle Fusion Cloud
The process of bringing a new pharmaceutical drug to market is a complex undertaking. This journey, known as drug commercialization, involves a meticulous series of steps from regulatory approval and manufacturing to marketing and final launch. Success demands precision, strict compliance, and operational efficiency. For organizations in the life sciences sector, leveraging a powerful Enterprise Resource Planning (ERP) system is not just an advantage—it’s essential.
Oracle Fusion Cloud provides an integrated suite of applications designed to meet the unique challenges of pharmaceutical manufacturing. It streamlines complex supply chains, ensures regulatory adherence, and provides real-time visibility into every stage of the commercialization process. This article details how Oracle Fusion Cloud ERP solutions empower pharmaceutical companies to navigate the path from clinical trials to full-scale commercial production, enhancing efficiency and accelerating time-to-market.
Understanding the Pharmaceutical Manufacturing Process
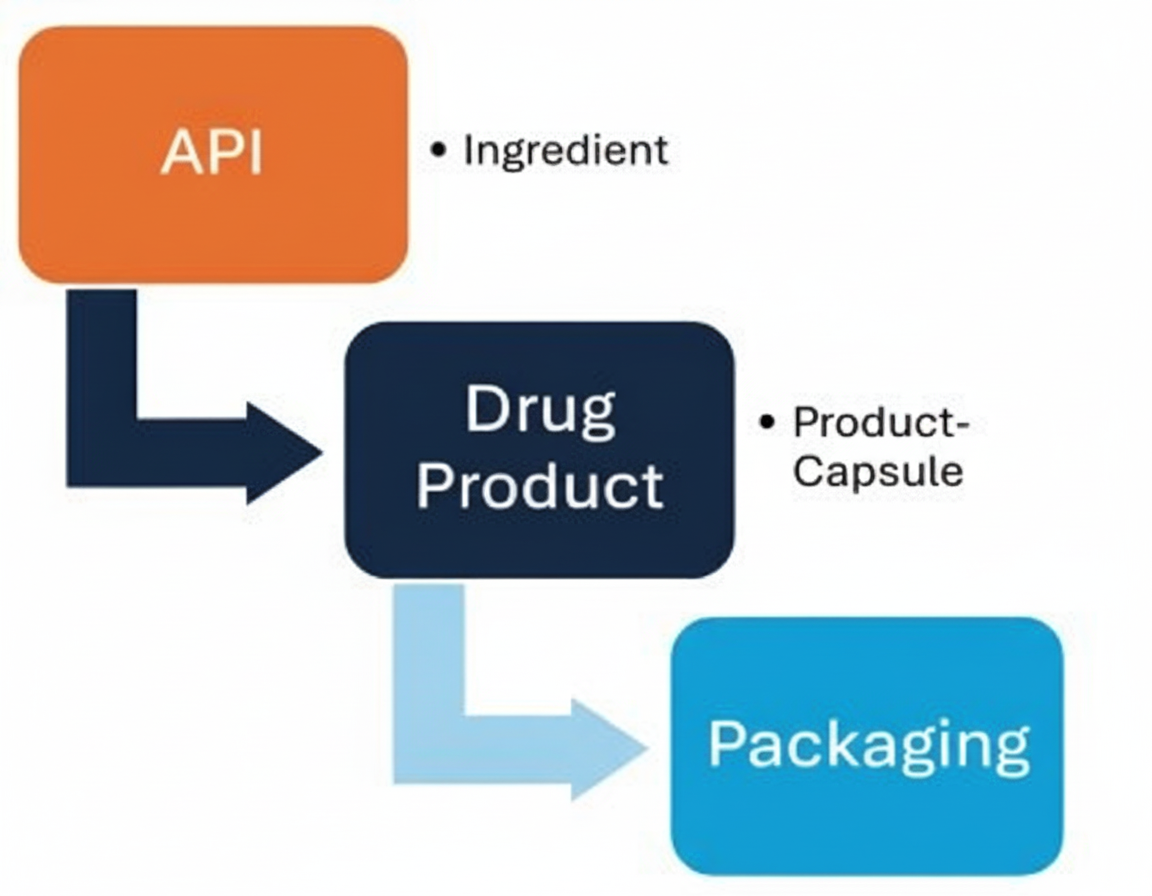
The pharma drug manufacturing process includes several manufacturing steps, starting with raw material procurement and quality control, followed by processing and purification to create the active pharmaceutical ingredient (API). Using API, Drug Product gets formulated and produced through processes like granulation, blending, and compression into dosage forms, which are then coated.
Each stage is governed by strict quality control measures, rigorous testing, packaging protocols, and distribution logistics, all conducted under Good Manufacturing Practice (GMP) guidelines. To manage this complexity, the industry relies on advanced software like the Oracle ERP suite to track, process, and account for every detail.
 |
 |
How Oracle Fusion Cloud Optimizes the Supply Chain
Oracle Fusion Cloud offers a comprehensive platform with modules for Supply Chain Management (SCM), Finance, and more, facilitating the digital transformation of pharmaceutical operations. The SCM module is particularly crucial, providing solutions tailored to the industry’s intricate requirements for forecasting, planning, product development, procurement, manufacturing, and costing.
A key feature is its ability to integrate seamlessly with finance applications, creating a unified system for tracking operations and their financial impact. This unified approach helps manage supply chain and procurement processes while ensuring complete tracking and traceability of lots across research and development (R&D), clinical trials, and commercial production streams.
The supply chain module provides an inbuilt Contract Manufacturing Process (CMP), addressing the pharma industry requirement for forecasting, planning, product development, procurement, manufacturing, costing, selling and integrate seamlessly with finance application.
This Oracle application helps manage supply chain and procurement processes, while ensuring tracking and traceability of lots across trial, R&D and Commercial process streams.
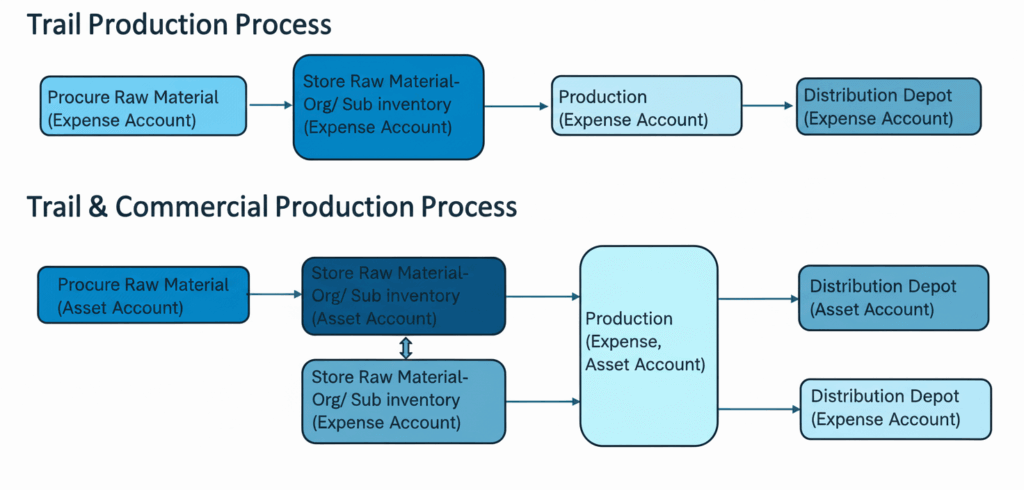
In CMP, the company ships raw materials to their vendor and manages the stock in the warehouse by configuring the vendor as an internal warehouse in SCM. This gives the vendor complete control of the manufacturing process and provides updates on inventory levels, complete work orders, and communications with the company. The company in turn processes the batch in ERP and makes payments. The following image (Fig 2) illustrates the business flow for contract manufacturing (1).

Driving Drug Commercialization
The drug manufacturing process involves defining new drug items based on drug composition (structure) and formula (process routing). With commercialization, industries will repurpose the in-stock trial raw material, API and drug products and use it for commercial production. The manufacturing processes, material and product tracking, and financial accounting must be enhanced and adapted to facilitate commercialization, while supporting trial and R&D activity.
Apps Associates designs and configures Oracle Cloud ERP solutions to help pharmaceutical clients manage this transition effectively. A well-designed solution addresses several critical areas:
- Warehouse and Inventory Management: Configuring warehouses to segregate and stock materials for trial and commercial use, ensuring no crossover and maintaining inventory integrity.
- Manufacturing and Costing Adjustments: Modifying work definitions and costing structures to accurately capture new overhead costs associated with commercial-scale production.
- Financial Tracking and Reporting: Designing and configuring Subledger Accounting (SLA) rules to precisely track the costs and revenues of trial versus commercial production and sales, providing clear financial insights.
Future technology in Business operations
Oracle Cloud provides the tools to aggregate and analyze data, enabling pharmaceutical companies to gain real-time insights and apply AI solutions for efficient operation and control. 
Key Benefits of Oracle Fusion Cloud for Pharma
Adopting an enhanced IT infrastructure with Oracle Fusion Cloud delivers tangible benefits for pharmaceutical and life sciences companies.
- Accelerated Speed-to-Market: An integrated and scalable solution helps streamline the complex processes involved in developing and launching new drugs, reducing delays and expediting market entry.
- Ensured Regulatory Compliance: Oracle’s applications include built-in features like audit trails and electronic signatures, which are essential for meeting stringent regulatory requirements such as the FDA’s 21 CFR Part 11.
- Improved Cost Efficiency: Automating supply chain management and procurement through a cloud-based system leads to significant cost savings and streamlined operations.
- Enhanced Financial Visibility: Real-time visibility into the financial performance of all operations allows for better strategic planning and resource allocation.
The journey of drug commercialization is fraught with logistical, regulatory, and financial complexities. Oracle Fusion Cloud provides a powerful, integrated ERP solution that empowers pharmaceutical companies to master these challenges. By optimizing supply chain management, ensuring compliance, and providing deep operational insights, Oracle helps businesses accelerate innovation and bring life-saving treatments to patients faster.
Partnering with an expert implementation provider like Apps Associates ensures that your organization can unlock the full potential of Oracle Fusion Cloud. Our team helps tailor the solution to your specific needs, guiding you through the transition from clinical trials to a successful commercial launch and beyond.
References
- Oracle Fusion Cloud SCM-Implementing Manufacturing and Supply Chain Materials Management-G34517-02
- The Future of Pharmaceutical Manufacturing Sciences
- Adobe Stock-photo
